Chemistry Isotopes Worksheet
Chemistry Isotopes Worksheet - The way to represent an isotope is to write the chemical symbol (or the word) followed by a dash and then the mass number. Many elements have a number of isotopes. The numbers 12, 13, and 14 refer to the mass number. Here are three isotopes of an element: 2) the atom with 17 electrons and 18. Grade 8, grade 9, grade 10, grade 11,.
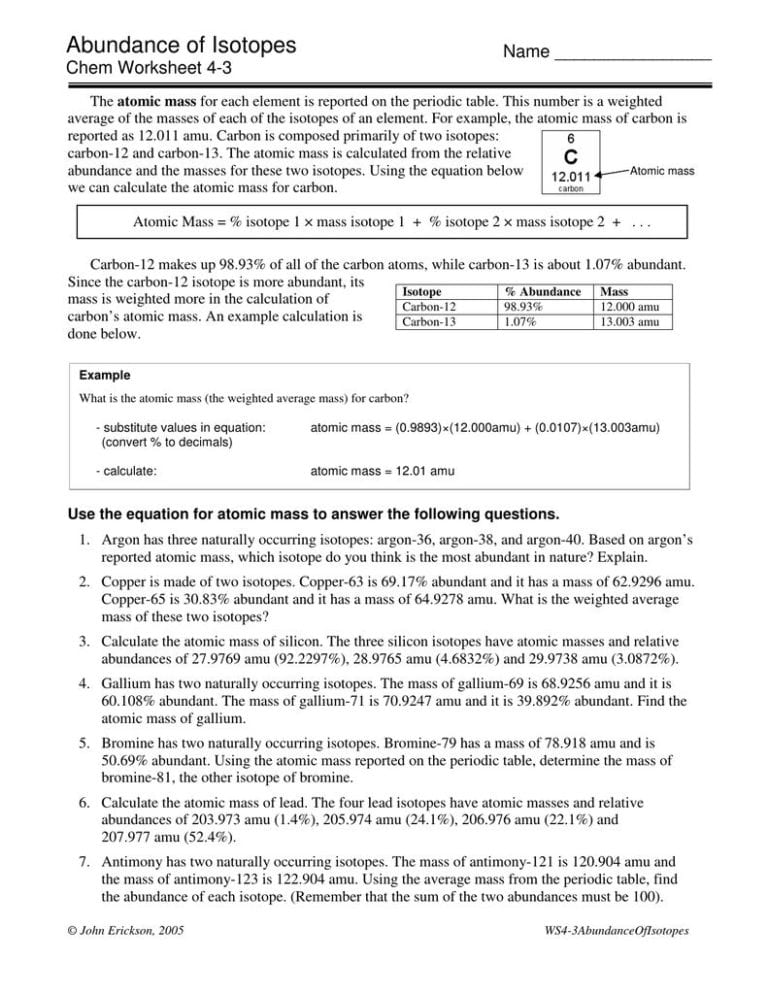
Calculate the atomic mass of an element which has two isotopes with masses of 12 (98.9%) and 13 (1.1%). Based on argon’s reported atomic mass, which isotope exist as the most abundant in nature? Grade 9, grade 10, grade 11,. The way to represent an isotope is to write the chemical symbol (or the word) followed by a dash and then the mass number. _____ bromine has two major isotopes giving it an atomic mass of 79.904 amu.
How do atoms become isotopes? How do atoms become cations and. Calculate the atomic mass of an element which has two isotopes with masses of 12 (98.9%) and 13 (1.1%). Fill in the isotope names and any missing information, including isotope numbers from the chart. _____ bromine has two major isotopes giving it an atomic mass of 79.904 amu.
How do atoms become cations and. For the following problems, show your work! The way to represent an isotope is to write the chemical symbol (or the word) followed by a dash and then the mass number. The number 6 refers to the atomic number. Calculate the atomic mass of an element which has two isotopes with masses of 12.
Write complete symbols (a symbol) for the following: _____ bromine has two major isotopes giving it an atomic mass of 79.904 amu. Magnesium consists of three isotopes with. (22) calculate the average relative atomic mass of ni to two decimal places. The number 6 refers to the _________________________ c.
The way to represent an isotope is to write the chemical symbol (or the word) followed by a dash and then the mass number. Fill in the isotope names and any missing information, including isotope numbers from the chart. Calculate the atomic mass of an element which has two isotopes with masses of 12 (98.9%) and 13 (1.1%). Answer the.
Identify the neutral atom described by name and mass number (i. Here are three isotopes of an element: For each of the following isotopes, write the # of protons, neutrons, and electrons. These worksheets will help students grasp the essential principles behind isotopes and learn how to distinguish between different isotopes. Isotopes are essential in various fields, including chemistry, physics,.
Chemistry Isotopes Worksheet - 2) the atom with 17 electrons and 18. Here are three isotopes of an element: The way to represent an isotope is to write the chemical symbol (or the word) followed by a dash and then the mass number. The number 6 refers to the _________________________ c. Identify the neutral atom described by name and mass number (i. These chemistry worksheets will give students a chance to practice a variety of problems and.
The way to represent an isotope is to write the chemical symbol (or the word) followed by a dash and then the mass number. Show your work and name the element. How do atoms become isotopes? Many elements have a number of isotopes. Calculate the atomic mass of an element which has two isotopes with masses of 12 (98.9%) and 13 (1.1%).
Here Are Three Isotopes Of An Element:
However, the two species differ in their proton number and atomic mass. The number 6 refers to the atomic number. _____ bromine has two major isotopes giving it an atomic mass of 79.904 amu. _________________ how many protons and neutrons are in the second.
The Numbers 12, 13, And 14 Refer To The Mass Number.
Grade 8, grade 9, grade 10, grade 11,. Argon has three naturally occurring isotopes: The number 6 refers to the _________________________ c. An isotope is an atomic species with the same atomic number as another species belonging to the same element.
Isotopes Are Essential In Various Fields, Including Chemistry, Physics, Medicine, And Environmental Science.
(22) calculate the average relative atomic mass of ni to two decimal places. For the following problems, show your work! Write complete symbols (a symbol) for the following: 12c 6 13c 6 14c 6.
For Each Of The Following Isotopes, Write The # Of Protons, Neutrons, And Electrons.
In this worksheet students can practice using the atomic number and reinforce isotopes by adding. Fill in the isotope names and any missing information, including isotope numbers from the chart. What does the number next to isotopes signify? Many elements have a number of isotopes.